Health
Promising at-home coronavirus test results in 15 minutes
WHAT YOU NEED TO KNOW:
- Biotech company Cellex in partnership with computer startup company Gauss are developing a COVID-19 test that can be performed with the use of a smartphone app.
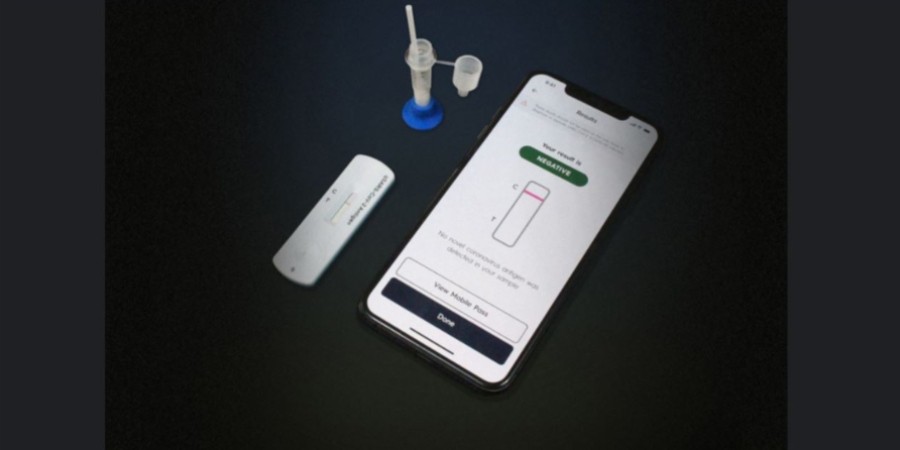
- The rapid-response test which can generate results in 15 minutes involves dipping nasal samples in buffered solutions then placed in a test cassette to be read by an AI.
- If approved by the FDA, the tool, which will be the first to be done at home, will allow people to self-monitor.
A new COVID-19 test that uses a smartphone app can deliver results within 15 minutes, making it the first test that can be done at home.
Axios reported that the rapid-response test which uses artificial intelligence to supply the results is being developed by biotech company Cellex in collaboration with Gauss, a computer vision startup.
Instead of needing a blood draw to process a person’s nose swab as performed in a laboratory, the antigen test involves taking a nasal sample from both nostrils then placing it in a small bottle containing a buffer solution. From the bottle, four droplets are taken and wiped on a test cassette.
Similar to a pregnancy test, lines will form with varying degrees depending on the amount of virus, if any, present in the sample. Axios explained that the sample is then scanned with a smartphone app that comes with an AI that will notify users if their results are positive or negative.
“By embedding advanced computer vision algorithms within a thoughtfully-designed user experience, we can enable consumers to perform a rapid test in their own homes just as well as a trained operator or a laboratory instrument — simply by using their smartphone cameras,” said Gauss CEO Siddarth Satish in a statement.
If the test gets approval from the FDA, it would be the first COVID-19 test that can be performed at home without needing a laboratory.
According to James Li, CEO of Cellex, the diagnostic has 90 percent sensitivity and almost 100 percent specificity.
Test sensitivity measures how often a test correctly identifies positive results while test specificity measures the test’s ability to correctly generate negative results. This means the rapid-test is not always 100 percent accurate in spotting people with COVID-19 but is always nearly accurate in detecting when a person is not infected.
He told Axios that with this tool, people can self-monitor and self-isolate when managing the COVID-19 crisis.
The test is slated later this year to be submitted to the FDA for Emergency Use Authorization.
Source: New York Post
